The site selection workflow. From CDA to decision.
Negotiate CDAs, run feasibility, and site decisions in one workflow — with every interaction (email, clause, response) captured into reusable site and contact records, so teams stop re-asking and start moving faster.
“Everyone is more productive with Yendou. We accelerated feasibility timelines by 50% while reducing manual follow-ups by 70%.”
VP of Solutions @ Lindus Health
.
⭐⭐⭐⭐⭐ Trusted by teams who can’t afford slow start-up

70% fewer manual touchpoints per feasibility per site

91% of sites complete feasibility tasks in under 5 days

2-4x increase in RFP throughput per FTE per week

1,800+ weekly active client–site engagements

12x more data points per site, up to 185 structured data fields

8+ hours saved per FTE per week
The Problem: Site selection still run on surveys, inbox threads, spreadsheets, and scattered systems.
In multi-center trials, that “artisanal” way of working creates three predictable failures:

Rework and manual follow-up slow timelines
Teams keep asking the same feasibility questions and chasing sites for replies, because engagement lives in email and spreadsheets instead of being captured once and reused.

Lost context when teams change or studies end
Site context lives in inboxes, spreadsheets, and people’s heads. When teams change or a study ends, it disappears, and your CTMS is already out of date.

Contracting & decisions disappear into threads
Agreements, revisions, and who’s blocking what are buried in inbox threads, making negotiations slow, opaque, and hard to manage across stakeholders and sites.
The Solution: Site engagement that becomes institutional memory
Yendou captures site responses, documents, and agreement history once—then reuses it across studies. So each new study starts with better data, faster outreach, and fewer touchpoints.
What you can do in Yendou
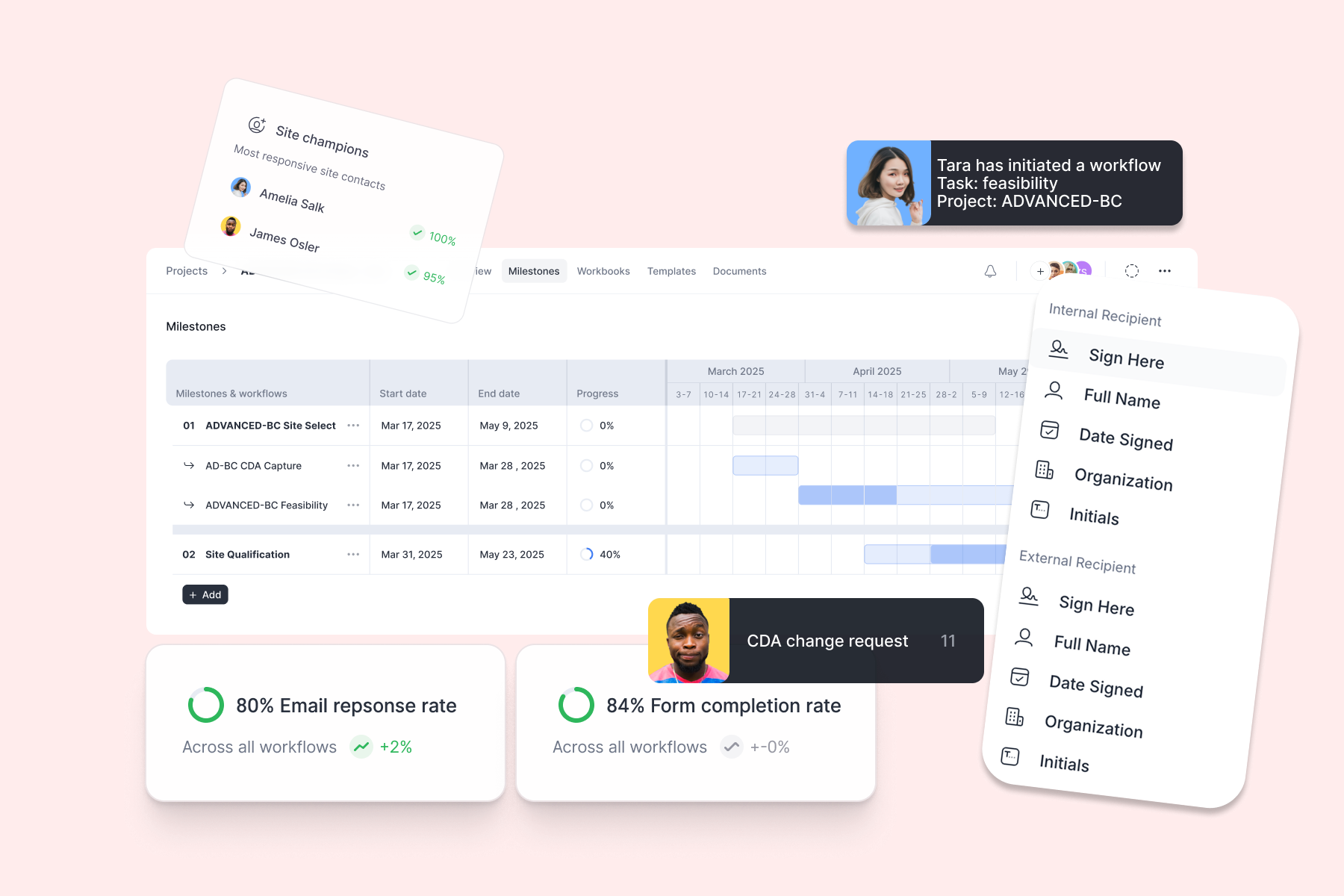
Make your site list smarter; automatically.
⦁ Bring your own site list, or start from Yendou’s database
⦁ AI-enrich every record (sites, people), and keep it searchable
⦁ Keep records continuously updated as your team and sites interact
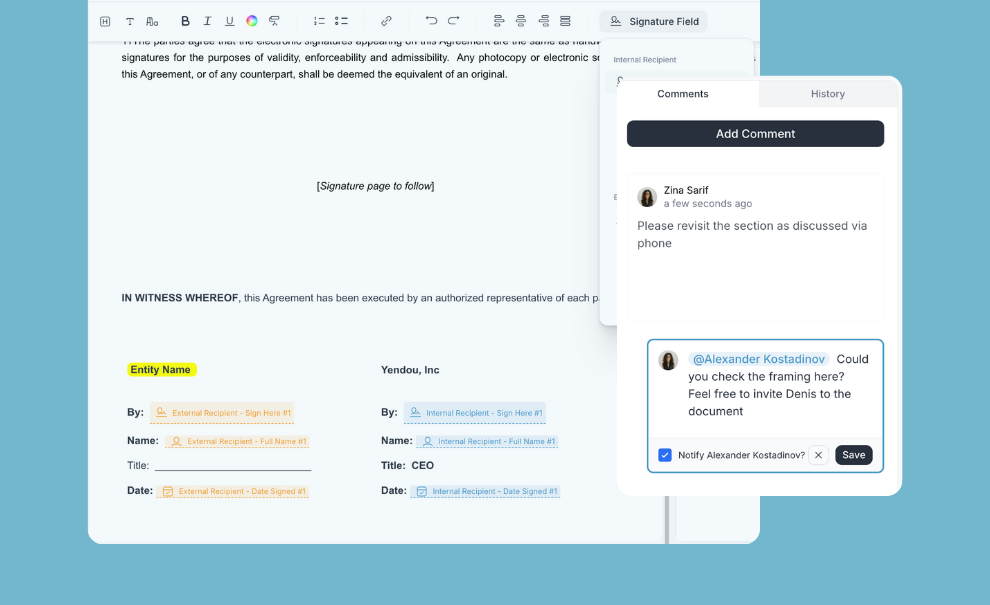
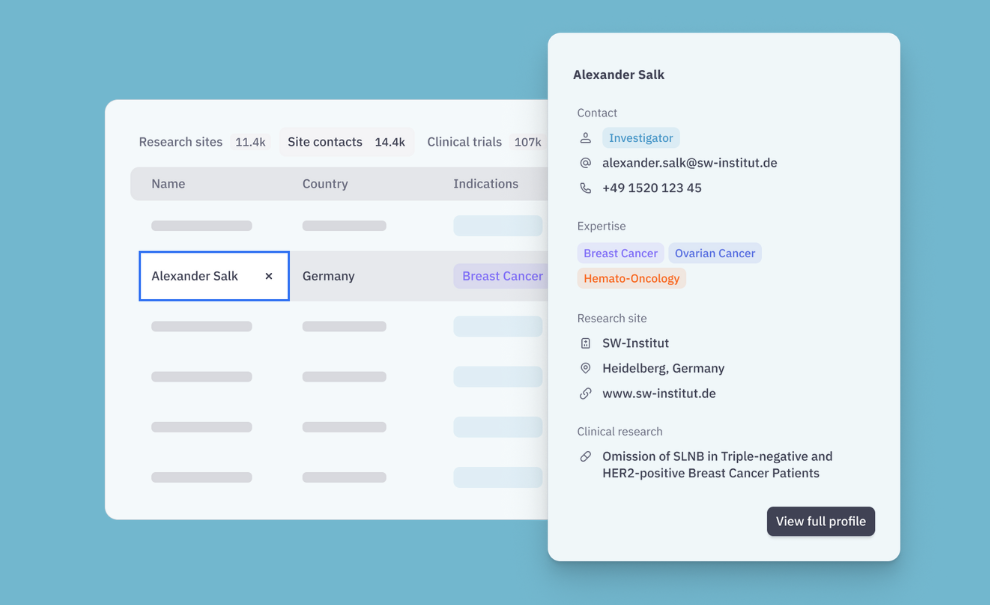
Send, negotiate, and sign CDAs without portal friction
⦁ Negotiate at scale directly with sites or site networks
⦁ Keep all versions, comments, and milestones in one place
⦁ No site login required for the engagement flow
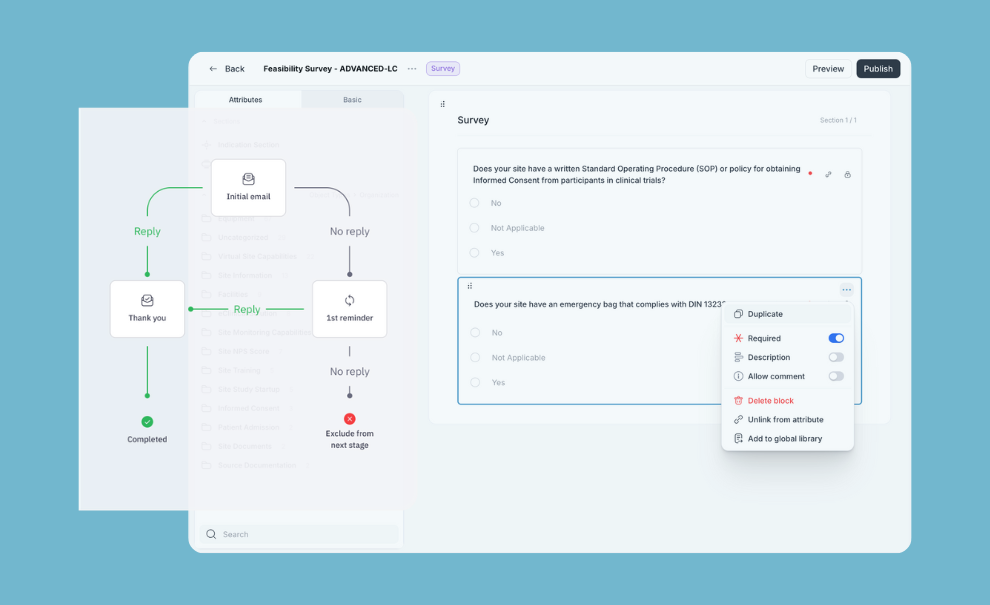
Run feasibility with zero repetitive work
⦁ Engage sites at scale directly (no site login) and keep the thread in one place
⦁ Turn replies into structured site records you can reuse across studies
⦁ Auto-track follow-ups and responsiveness so nothing slips
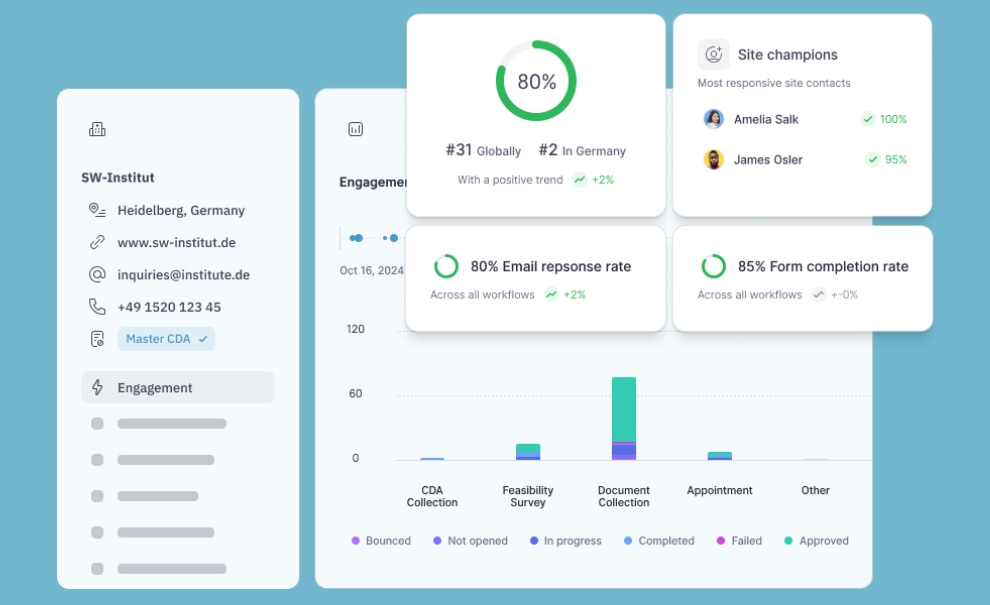
Measure site responsiveness
⦁ Automatically track how long sites take to respond and complete tasks
⦁ Identify “champion” sites and reliable site networks
⦁ Benchmark sites by speed and quality of information shared
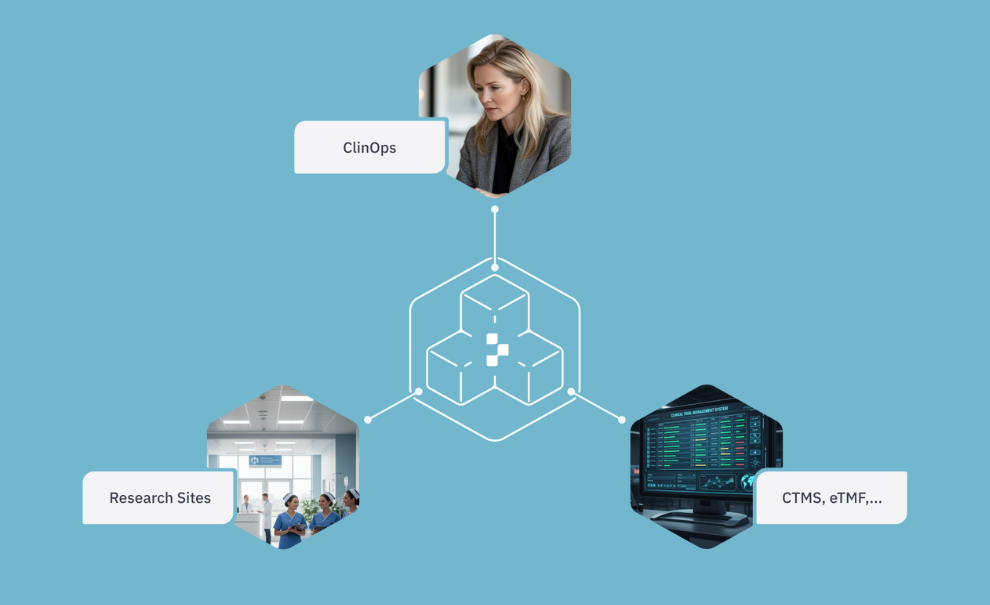
Keep systems aligned (CTMS & eTMF integration)
⦁ Bi-directional sync of sites, contacts, documents, agreements, and tasks
⦁ Updates in Yendou reflect back to CTMS, so information is entered once
⦁ Sync rules ensure only what you approve is written back
How Yendou works (in 5 steps)
Step 1: Identify
sites and contacts (upload or search)
Step 2: Engage
in feasibility + agreements workflows (No site logins)
Step 3: Capture
every answer and milestone into the site record
Step 4: Sync
approved data back to yendou and external systems (CTMS/eTMF)
Step 5: Reuse
what you’ve already learned, so the next study starts with a smarter site list, fewer questions, and fewer follow-ups.
Results (not promises)

Lindus Health
[CRO | 200FTE | 94% FTE Growth in 2025]
⦁ 70% fewer site follow-ups
⦁ 50% faster feasibility timelines
⦁ 39 workdays saved per FTE per year
⦁ 84% of sites complete tasks within five days
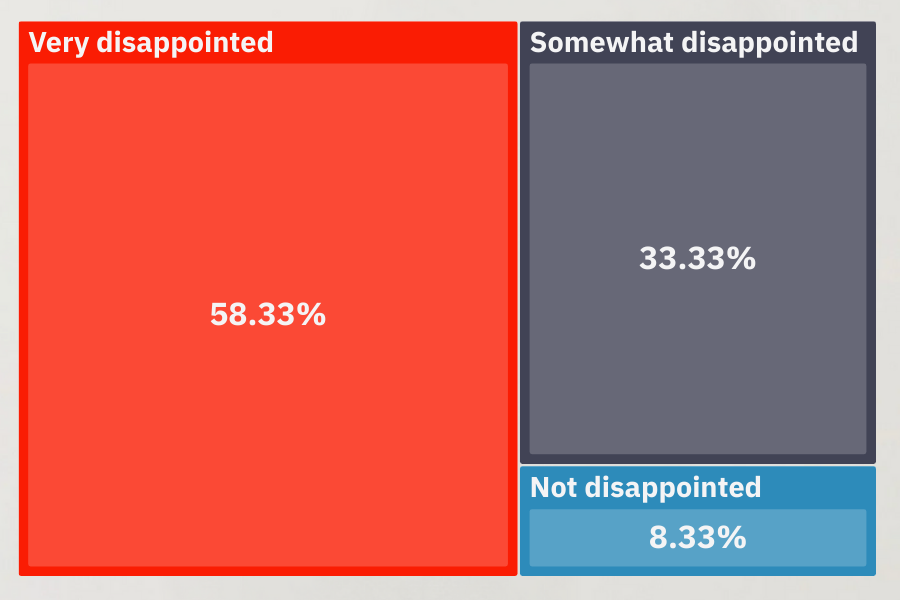
Site Sentiment
[163 Sites | US, UK, EU, Asia | December 2025]
We asked Sites: “If a sponsor/CRO stopped using Yendou for feasibility and went back to the previous way of working, how would you feel?”
"At Lindus Health, we've seen that when sites feel valued as strategic partners, everything changes—trust deepens, loyalty strengthens, and response times accelerate, Partnering with Yendou helps make that possible."
Meri Beckwith | co-CEO @ Lindus Health
91% of sites complete feasibility within five days
”Getting surveys pre-filled is a game changer. We’re not wasting time answering the same questions again and again.… that is very cool.”
“My experience? Well, it was really easy. You know, I was able to click, click, click and see everything. It was. It’s very nice. It’s very clean. It’s got a very nice overview. I really like it.”
"It’s great working with you guys. you’re offering site networks that level of control and comfort we never experienced before. We’re being notified when there’s opportunities and not worrying about our sites falling off a list. They won’t be forgotten.”
"This isn’t just another portal. As a site management organization, the performance tracking KPIs are genuinely impressive. Having the opportunity to gain market visibility and showcase your performance is highly compelling."
“Being able to download everything at once saves us so much time. We don’t have to screenshot or piece things together anymore. Beyond that, it’s simple, intuitive, and just really well designed. ... I love it the way it is…”
“The Platform takes a lot of the workload off our site teams. We can finally handle everything in one place.
“What Yendou is doing is exactly what sites have been waiting for. A way to stay connected and aligned. It keeps us up to date, and we can easily share changes, like new PIs or therapeutic areas. It helps both sides stay informed.”
"This is something CROs really need. Improving communication with sites is often overlooked, and this platform tackles it head-on."
Security, privacy, and compliance

User and study-level permissions

Import/export/delete account and activity data
GDPR-aligned data residency, including EU data stored in Germany

Operational safeguards: uptime targets, backups, recovery procedures
Built for clinical operations reality

Pre-award feasibility teams
Get more proposals out faster, and move more deals to the bid stage with better pre-award feasibility. Teams using Yendou run 4× more pre-award feasibilities per week.

Feasibility teams
Build site lists faster, reduce site follow-ups using feasibility workflow templates, and eliminate repetitive questionnaires by reusing known site answers across studies.

Contracting teams
Track every redline, comment, and approval in one place. Create, and negotiateCDAs across multiple sites in parallel, while every version is saved automatically with a complete audit log. 21 CFR Part 11 compliant.
Frequently Asked Questions
Is Yendou “just” a feasibility tool?
No. Feasibility is one workflow. Yendou is the system that turns site engagement into reusable site intelligence across feasibility, agreements, and SSU.
Do sites need a portal account?
No—sites can engage without logging into a separate platform.
How is Yendou’s site intel different from “reported site performance”?
Reported performance is self-claimed or manually tracked. Yendou measures responsiveness and task completion from real engagement activity, so you can select sites using measured performance, not self-reported claims.
How is Yendou’s site intel different from market benchmark data?
Market benchmarks tell you what’s typical for companies like yours. Yendou tells you what’s true for you—by measuring how fast each site responds and completes tasks when working with your team.
How does CTMS sync work?
Yendou syncs sites, contacts, documents, agreements, and tasks bi-directionally, with rules that control what’s written back.
Insights to Shape Your Next Breakthrough
Make feasibility and SSU execution repeatable; without repeating the work.
Book a demo to see Yendou’s site search, engagement workflows, and reusable site records in action.
















